


|
 |
Fall 2021 updates: I recently published a couple papers, linked below. The first is a solo-author manuscript in Journal of Petrology in which I present a series of calculated redox-sensitive fluid speciations governed by silica activity during serpentinization. The second is a collaboration with Emily Cooperdock at USC and my grad student Bryan Seymour on whole-rock geochemical analyses of the New Idria serpentinite body, in which we report elemental signatures consistent with a forearc mantle origin, i.e., a subduction-derived origin. Oh, and also I was tenured and promoted to Associate Professor. So I can spend time doing fun stuff like starting a YouTube channel in collaboration with the CSUSB Faculty Entrepreneur Fellow program, developing some virtual reality crystallography content in collaboration with the CSUSB xREAL lab, and continuing to create mildly amusing memes and other performative info-tainment on my Instagram feed. Don't worry I won't be a total deadbeat researcher: I'll be continuing my work on New Idria, in addition to developing projects in the Canyon Mountain Complex, Oregon, resuming some challenging experimental simulations of abiotic alkanogenesis on planetary seafloors, and working with undergrads on trying to measure the solubility of hanksite, a triple-salt pain-in-the-ass evaporite mineral. |
 |
Subduction signatures in the New Idria serpentinite diapir New Idria is a large diapiric serpentinite massif in central California believed to have a deep subduction-derived origin. My co-authors and I tested this hypothesis with whole-rock elemental analyses of minor, and trace elements, and suggested that the results are consistent with a slab-derived fluid and a mantle wedge protolith, as would be expected in a subduction-derived serpentinte. We reported extremely high Cs and Ba concentrations: fluid mobile element enrichments strongly suggestive of a slab origin. Major element sugnatures were extremely depleted, as expected in the high melt-flux setting of the mantle wedge. Stay tuned for more work on this fascinating locality... [Lazar, Cooperdock and Seymour , 2021, Lithos, PDF] |
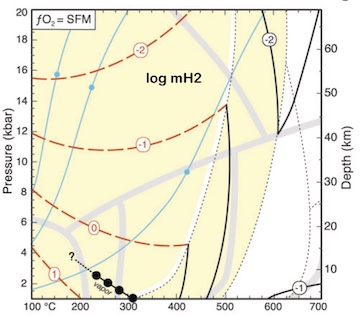 |
Using silica activity to model redox-sensitive fluids in subduction zones Building on the work by Frost and Beard, 2007, I used silica activity buffered by serpentinization reactions to compute redox-sensitive fluid compositions from 100–700ºC and 1–20 kbar. This is a pretty nerdy paper, so I'll just summarize the key result: reduced fluid species of carbon and sulfur are predicted over most of the possible P–T range for subducted serpentinites and in the mantle wedge. Subduction zones have great thermodynamic potential for the production of abundant reduced fluid species. [Lazar, 2020, Journal of Petrology , PDF] |
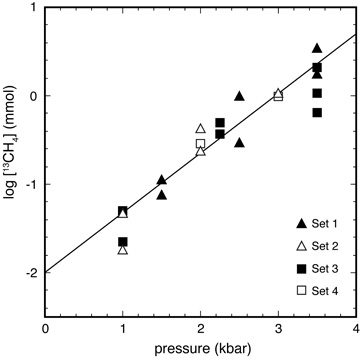 |
A kinetic pressure effect on methanogenesis Aqueous abiotic methane concentrations in a range of geologic settings are below levels expected for equilibrium with coexisting CO2 and H2, indicating that kinetics can control the speciation of reduced carbon-bearing fluids. Previous studies have suggested that mineral catalysts or gas phase reaction may increase the rate of methanogenesis. Here, we report experiments that indicate pressure can also accelerate hydrothermal aqueous reduction of CO2 to methane. The pressure effect was interpreted to be mediated by one of the following three processes: reduction of metastable methanol, formation of Fe-carbonyl complexes in the fluid, and/or heterogeneous catalysis by Fe. Pressure-enhanced methanogenesis may lead to CH4-rich fluids in the forearc regions of subduction zones. A kinetic pressure effect may be particularly important on the deep ocean floors of planetary bodies where pressure may compensate for the otherwise sluggish reaction kinetics expected at low temperatures. [Lazar et al. 2015, Geochimica et Cosmochimica Acta, PDF] |
 |
Calcite reduction in subduction zones Calcite is an important carrier of inorganic carbon in subduction zones. We show that calcite solubility increases with decreasing ƒO2 until a critical point below which portlandite is stable relative to calcite (LEFT). As depth increases, the enhancement of carbon mobility above oxidized values requires lower and lower ƒO2 values. For example, at the seafloor, QFM is sufficient to enhance carbon mobility, but at blueschist conditions only serpentinization and other very low ƒO2 processes can increase calcite solubility above oxidized conditions. Notably, we report a fascinating phenomenon wherein calcite melts at low ƒO2 in the presence of H2O at 700 °C and 10 kbar because the reduction of calcite to portlandite causes a freezing point depression. [Lazar et al. 2014, notable paper, American Mineralogist, PDF] |
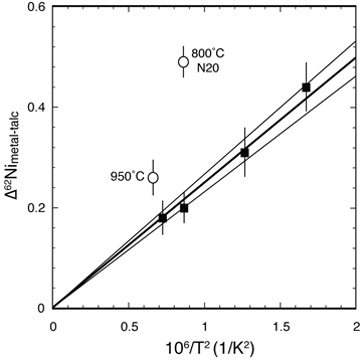 |
Experimental Ni isotope equilibrium Recent advances in mass spectrometry have led to exciting advances in nontraditional stable isotope geochemistry. Measurements in natural materials require high quality experimental calibrations of relevant fractionation factors. Combining the three-isotope method with classical experimental petrology, we measured the equilibrium Ni isotope fractionation factor between Ni-metal and Ni-talc (LEFT) as a model for metal-silicate fractionation in general. [Lazar et al. 2012, Geochimica et Cosmochimica Acta, PDF] |
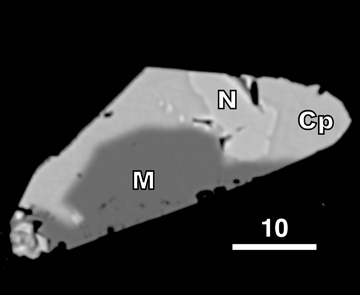 |
Greenhouse methane from komatiite alteration Greenhouse methane that formed during serpentinization of a komatiitic seafloor may have warmed the early Earth, possibly enough to prevent glaciation and solve the so-called Faint Young Sun paradox. To test this hypothesis, we measured methane yields during experimental hydrothermal alteration of komatiites. Ni-sulfides ("N", LEFT) were inferred to catalyze methanogenesis. Chromite, previously proposed as an alkane catalyst, was not catalytic. Modeling suggests that komatiite alteration alone could not have warmed the early Earth above freezing, although it may have had secondary climatic effects [Lazar et al. 2012, Chemical Geology, PDF] |
 |
Novel moderately-reducing experimental buffers A persistent problem in experimental petrology is the availability of buffering assemblages between QFM and IM or IW. We discovered an easy-to-use buffering assemblage that falls in the ƒO2 "blind spot": quartz-metal-willemseite, where willemseite is nickel talc (light blue-green at LEFT). Such a buffer should also exist in the cobalt system. Calibration of these buffers requires measurement of the standard state Gibbs free energy of the relevant talc phases. We performed this for willemseite with UCLA undergrad Mike Huh. |
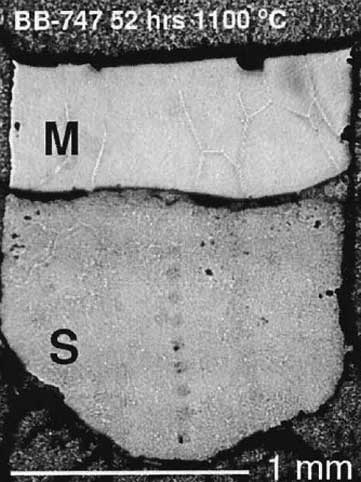 |
Tc-Mo-Ru partitioning in metal-sulfide melt Several authors reported molybdenum and ruthenium isotope anomalies in iron meteorites, suggesting that short-lived parent isotopes of technetium were active during core formation. Evaluation of whether such a process is feasible requires knowledge of the partitioning behavior of Tc, which was unknown until we measured it during my master's thesis work. The experiments (LEFT) showed that Tc partitioning between metal and sulfide melt was too modest to result in substantial fractionation during core formation. [Lazar et al. 2004, Geochimica et Cosmochimica Acta, PDF] |
back to top
Labs I've worked in
|
|
|
||||||
|
|
|
Here's some more images from my two years as an earth science teacher at Washington Irving High School.
Here's me on NPR in 2007 narrating the sounds of experimental petrology.
 Award-winning pumpkin depicting the spooky 2nd Law of Thermodynamics back to top |